Products
Description
Type 2 Diabetes Mellitus is a growing problem across the world. There is now strong evidence that intensive control of blood glucose can significantly reduce and retard the microvascular complications of retinopathy, nephropathy and neuropathy. However, upto 80% of Type 2 diabetics die from macrovascular cardiac disease.
For better patient compliance, Metformin is formulated in sustained-release form as GLYCIPHAGE SR® Tablets.
GLYCIPHAGE SR® comprises of a dual hydrophilic polymer matrix system. On administration, fluid from the gastrointestinal (GI) tract enters the tablet, causing the polymers to hydrate and swell. Drug is released slowly from the dosage form by the process of diffusion that is essentially independent of pH.
The biologically inert components of the tablet are eliminated in the feces as a soft, hydrated mass.
GLYCIPHAGE SR 500® GLYCIPHAGE SR 850 mg® GLYCIPHAGE SR 1 gm® TABLETS
(ORAL ANTI-HYPERGLYCEMIC AGENT)
Composition
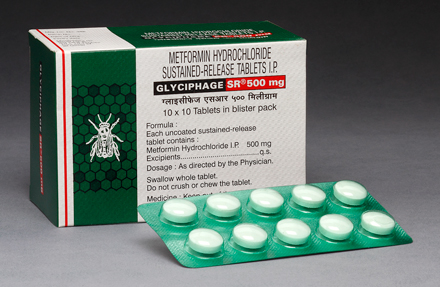
GLYCIPHAGE SR® 500 mg
Each uncoated sustained-release tablet contains:
Metformin Hydrochloride I.P. ....................................... 500 mg.
Excipients ..................................................................... q.s.
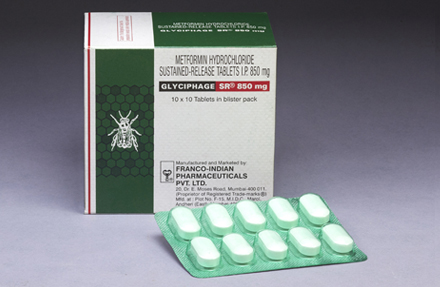
GLYCIPHAGE SR® 850 mg
Each uncoated sustained-release tablet contains:
Metformin Hydrochloride I.P............................................. 850 mg.
Excipients ..................................................................... q.s.
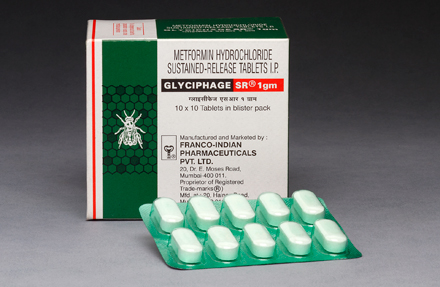
GLYCIPHAGE SR® 1gm
Each uncoated sustained-release tablet contains :
Metformin Hydrochloride I.P. ..........................................1 gm.
Excipients ..................................................................... q.s.
ORAL ANTI-HYPERGLYCEMIC DRUGS
ANTI-HYPERLIPIDEMIC DRUGS
ANTI-OBESITY
PAIN MANAGEMENT
ANTI-HYPERURICEMIC DRUGS
VITAMINS/MINERALS
LAXATIVE